|
Application for consultative and analytical support for registration
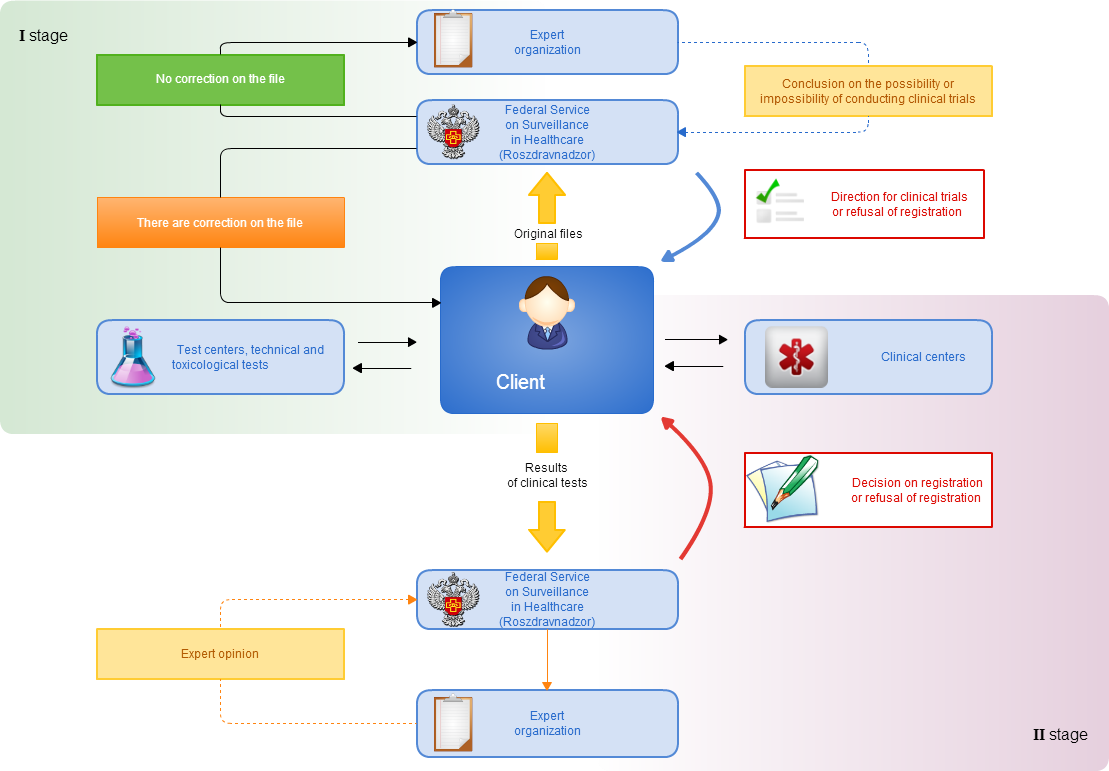
Stages of preparation of documents for registration of medical products of foreign production
I. Documents provided by the manufacturer of a medical device:
- Power of attorney from the manufacturer registered in the territory of the Russian Federation.
- Documents confirming registration of the manufacturer in the country.
- Documents confirming compliance with the conditions of production of medical products with international requirements: certificate / declaration of conformity of production conditions and quality requirements of the European Directive EC 93/42 (for reagents and analyzers CE 98/79), the certificate of conformity of production conditions the product requirements of ISO 9001 and / or ISO 13485.
- Photographic images of the general form of a medical device labeled, stamped and signed by the manufacturer.
- Technical documentation for the medical device containing the data:
- development (design and description of embodiments, an extract from TK);
- for the production of (a description of the required equipment and operating conditions);
- for use (where, why, in the (non) sterile, staff requirements);
- for operation (how: procedures);
- maintenance (indication (not) use, description, if applicable);
- for repair (a description of what the user can do, if that is unacceptable, specify contacts authorized representative in the Russian Federation or warranty service);
- for recycling or destruction (type and procedure).
- Operational documentation containing information for the user with a reference:
- design of medical products;
- regulatory conditions;
- operating rules:
- intended use,
- Maintenance,
- repairs,
- storage,
- transportation;
- guaranteed by the manufacturer values of key parameters, characteristics (properties) of the medical device;
- warranty;
- information about its recycling or destruction.
II. Analysis of the technical documentation for compliance with RF
- Application to the analysis of technical documentation.
III. Documents provided by the legal entity registered in the Russian Federation, the authorized representative of the manufacturer:
- Application for registration of MI (indicating the range of products in the form of articles or catalog numbers or sizes in the appendix to the application). *
- Table comparing the basic parameters of the registered product with the equivalent (in the absence of the table products are sent for clinical trials and examination of documents, which significantly increases the cost and time of registration). Application is made to the application.
- Power of attorney to submit documents - 1 copy.
IV. Testing
- To conduct technical tests (TI) and toxicological tests must submit the following documents (electronic and paper):
- Application Test
- application for registration MI - 2 copies.
- CE Declaration on the registered medical device with an official translation into Russian (matching the EC Directive 93/42 and CE 98/79).
- regulatory document - 2 copies.
- operational documentation - 2 copies.
- pictures in email. form of labeled products (general view and labeling).
- samples of products (number of samples in accordance with GOST 51148-98 Appendix B).
- For comparative analysis with previously registered products registered in the RF analogue ( search page registered in the RF analog) to provide comparison table with analog.
V. Copies of the charter documents of the organization:
- Documents confirming registration of the applicant organization as a legal entity:
- extract from the Unified State Register of Legal Entities (Entities).
VI. Options to pass to the case Roszdrav:
- Collection of documents specified in Sections I - V *
- Burn Disc (CD) with the electronic version of the following documents:
- application;
- reference;
- technical documentation;
- manual or instructions.
- Providing the original payment order for 6000 rubles. (State duty) or a copy of the blue print of the bank.
- Providing the original payment order for the examination of the registration dossier.
- Formation Inventory - 2 copies.
Upon receiving information about the readiness of the registration certificate is issued in the Agency for Health Care Power of Attorney for documents - 1 copy.
* These documents must be submitted in duplicate - in electronic (format Word) and paper formats
All documents of more than two (2) sheets are stitched and certified by signature of the head of the organization or person authorized by him.
Copies of all documents issued in accordance with GOST 6.30-2003 paragraph 3.26
List of documents required for the receipt of the letter on the import of medical devices for the purpose of registration
- Statement , signed by the head entity, which shall include:
- name of the medicinal product, indicating the configuration, number, serial number, lot number or batch number, date of manufacture of the medical device, its validity and (or) operation;
- appointment of a medical device manufacturer installed;
- full or abbreviated (if available) the name, legal form of the applicant, the address of its location, the state registration number of the record to establish a company, telephone number and (if available) e-mail address;
- information about organizations that planned technical testing, toxicology studies, clinical trials, as well as tests for approval of type of measurement (for health care products related to measuring instruments in the field of state regulation to ensure the uniformity of measurements).
- The application shall be accompanied by:
- copies of contracts to conduct the necessary tests (Research), specifying the number of medical devices;
- copy of the document certifying the authority of an authorized representative of the manufacturer
If you have questions on the processing and acquisition of documents for registration of products, please contact us at the numbers listed below. We will be glad to help.
Head: Shandova Nadezhda Pavlovna
tel. / fax: (495) 502-88-81, 502-88-82, 502-888-9
mob. tel.: (495) 921-14-55
Address: Ul. Pine Alley, 6, Korolev, Moscow region., 141075
|
